Of the approximately 73 million induced abortions that took place between 2015 and 2019, an estimated 24 million were unsafe. In line with current scientific data and the World Health Organization’s recommendations, Linepharma International aims to make safe abortion and sexual and reproductive healthcare accessible to as many people as possible, in as many places as possible, regardless of their social, political, or economic circumstances.
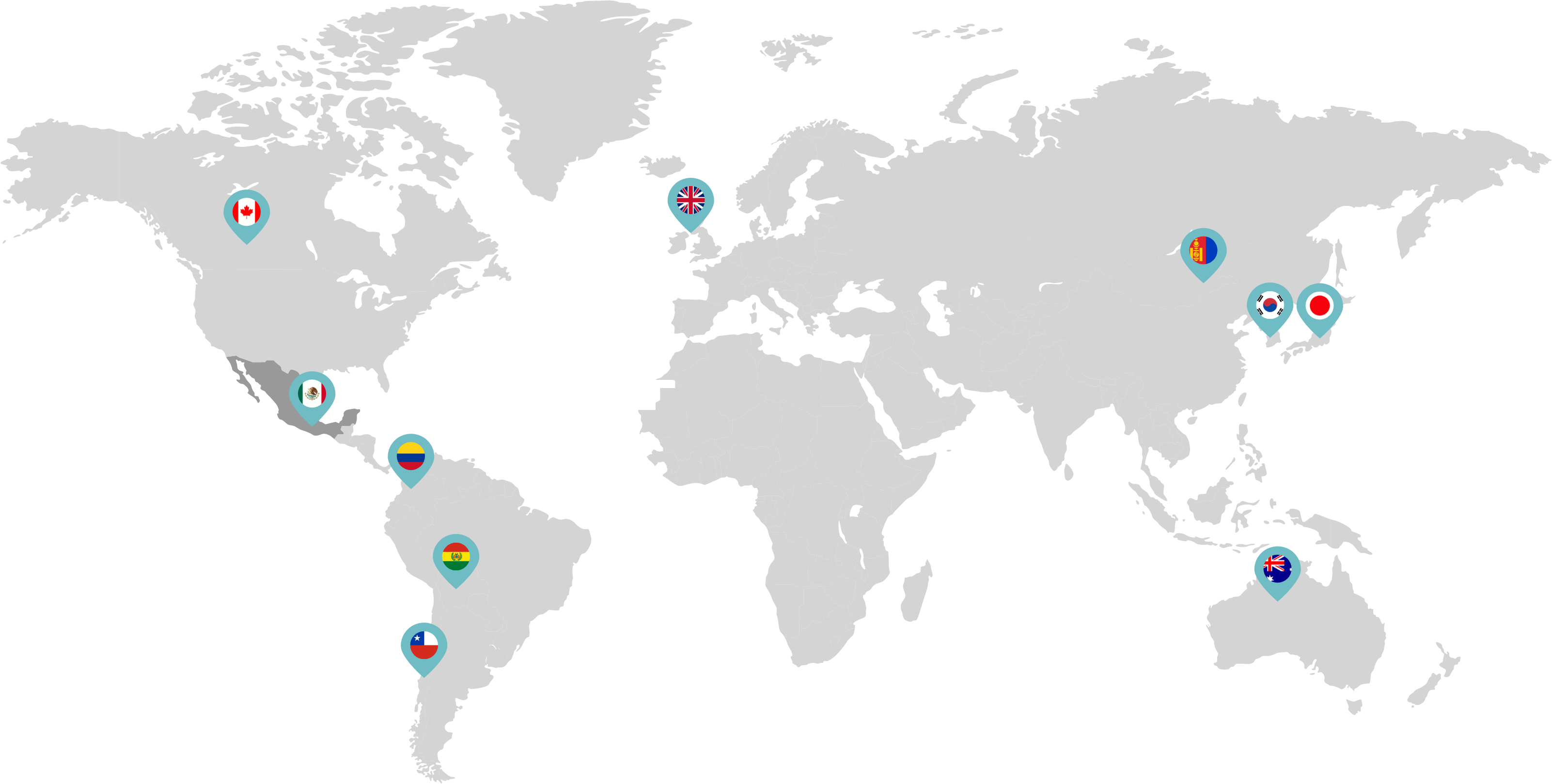
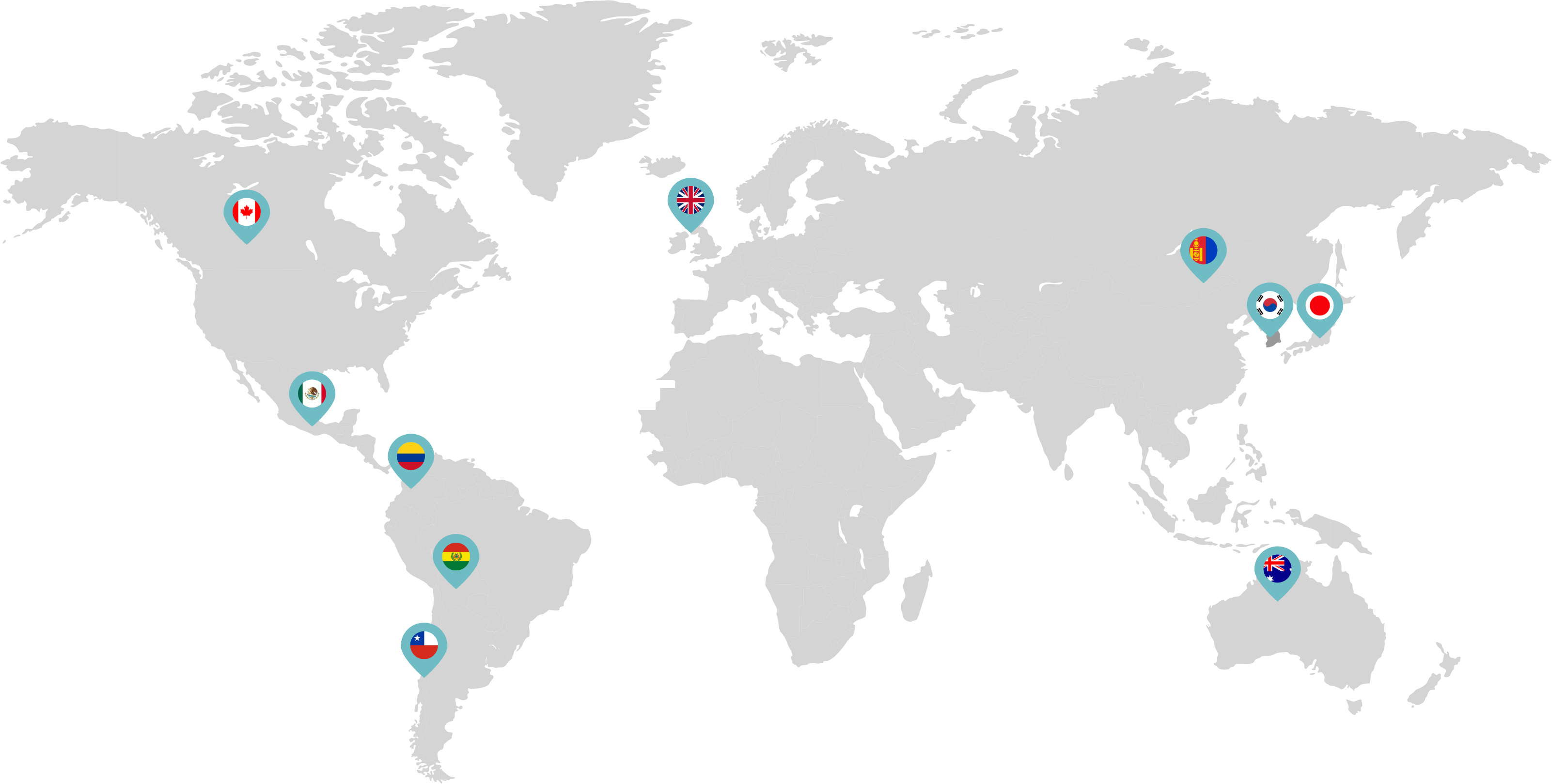
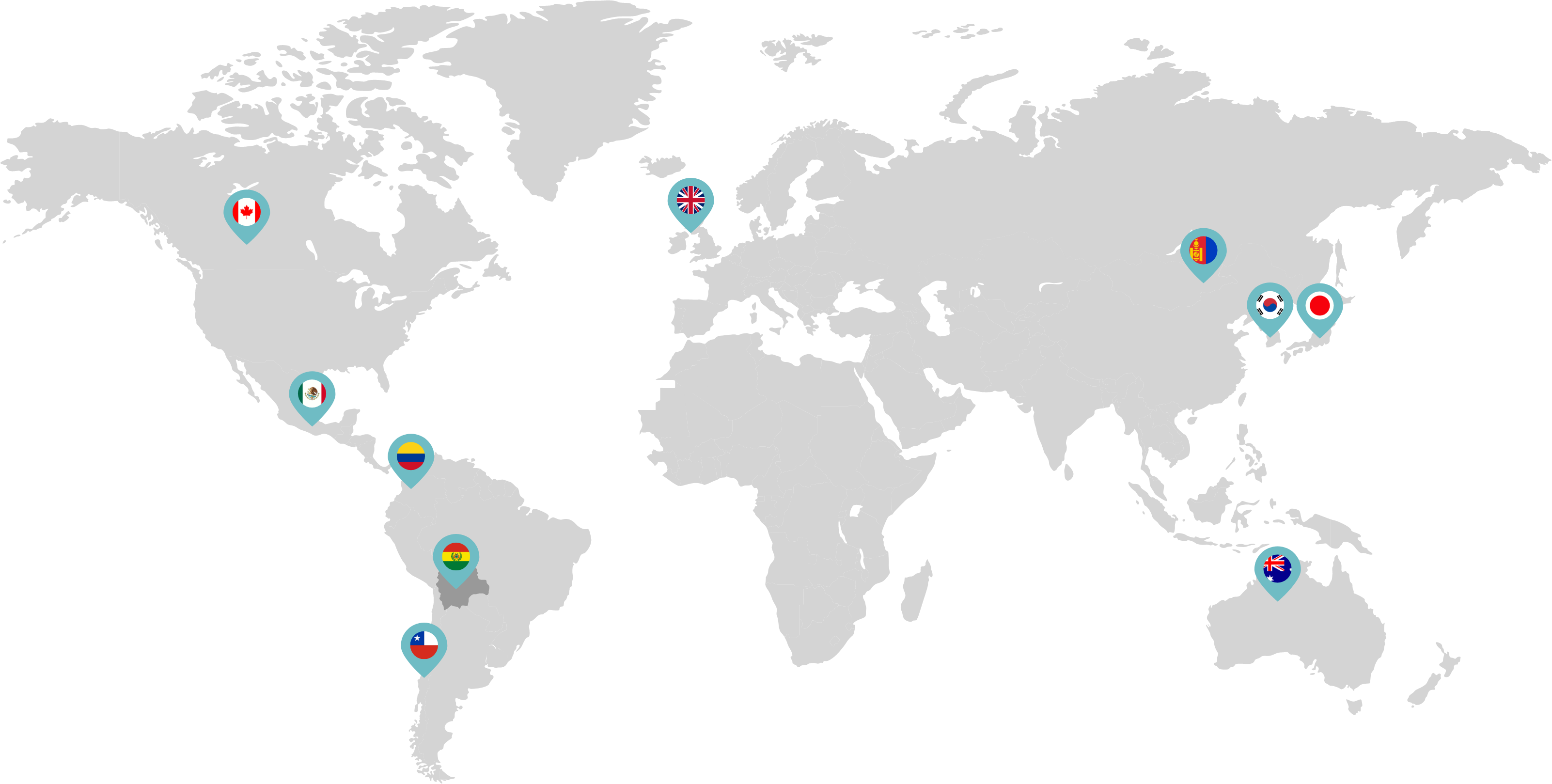
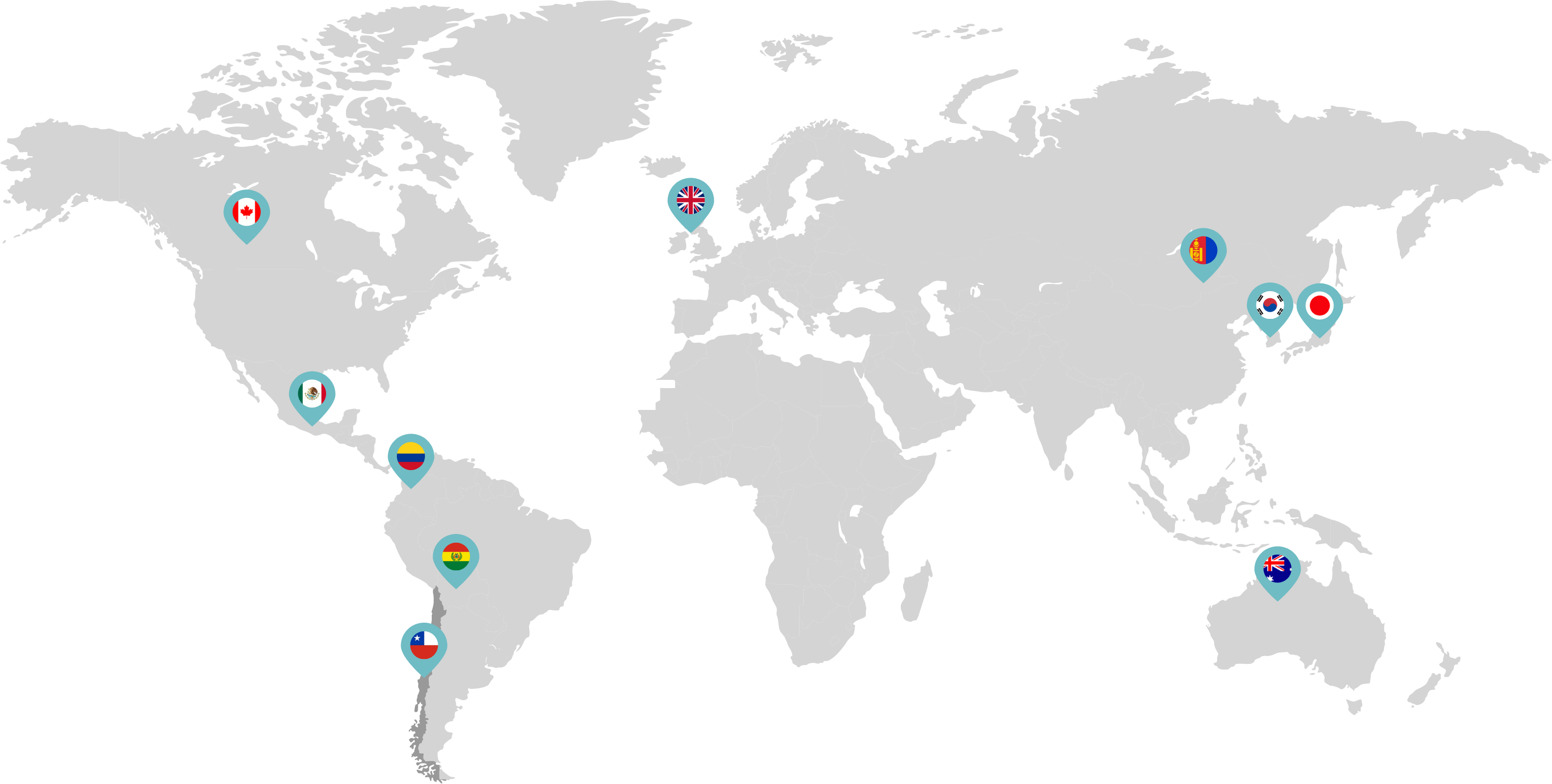
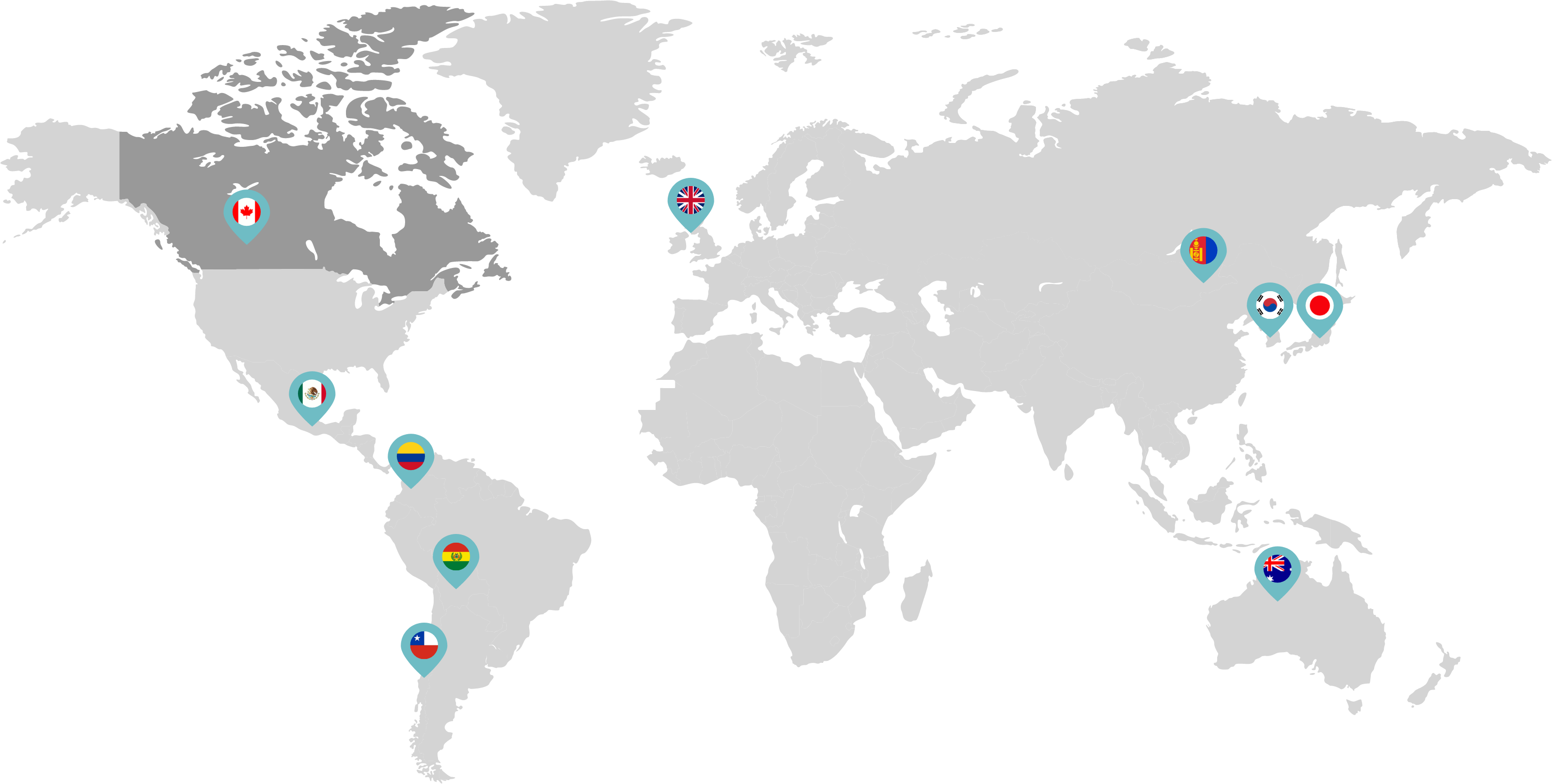
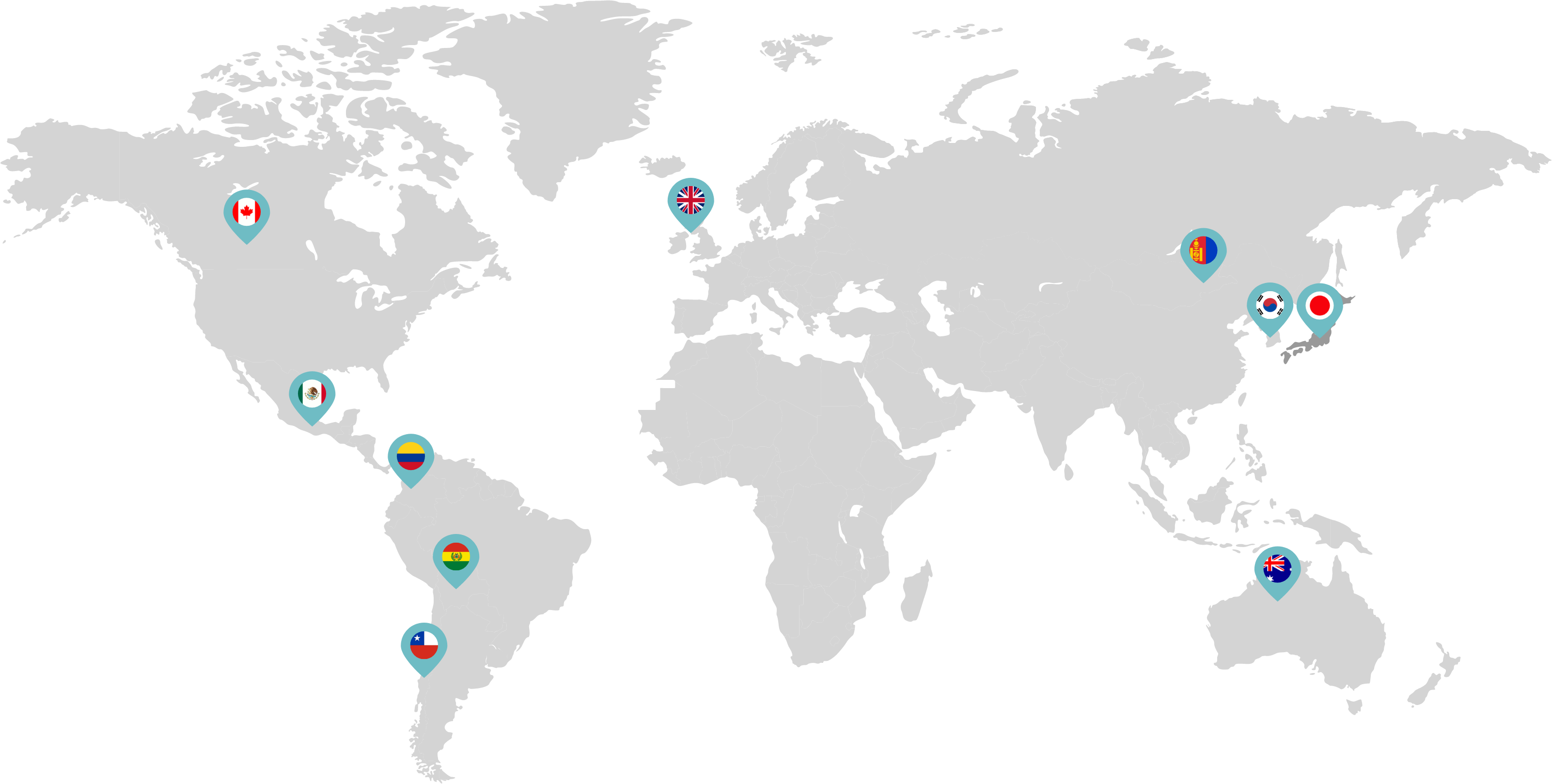
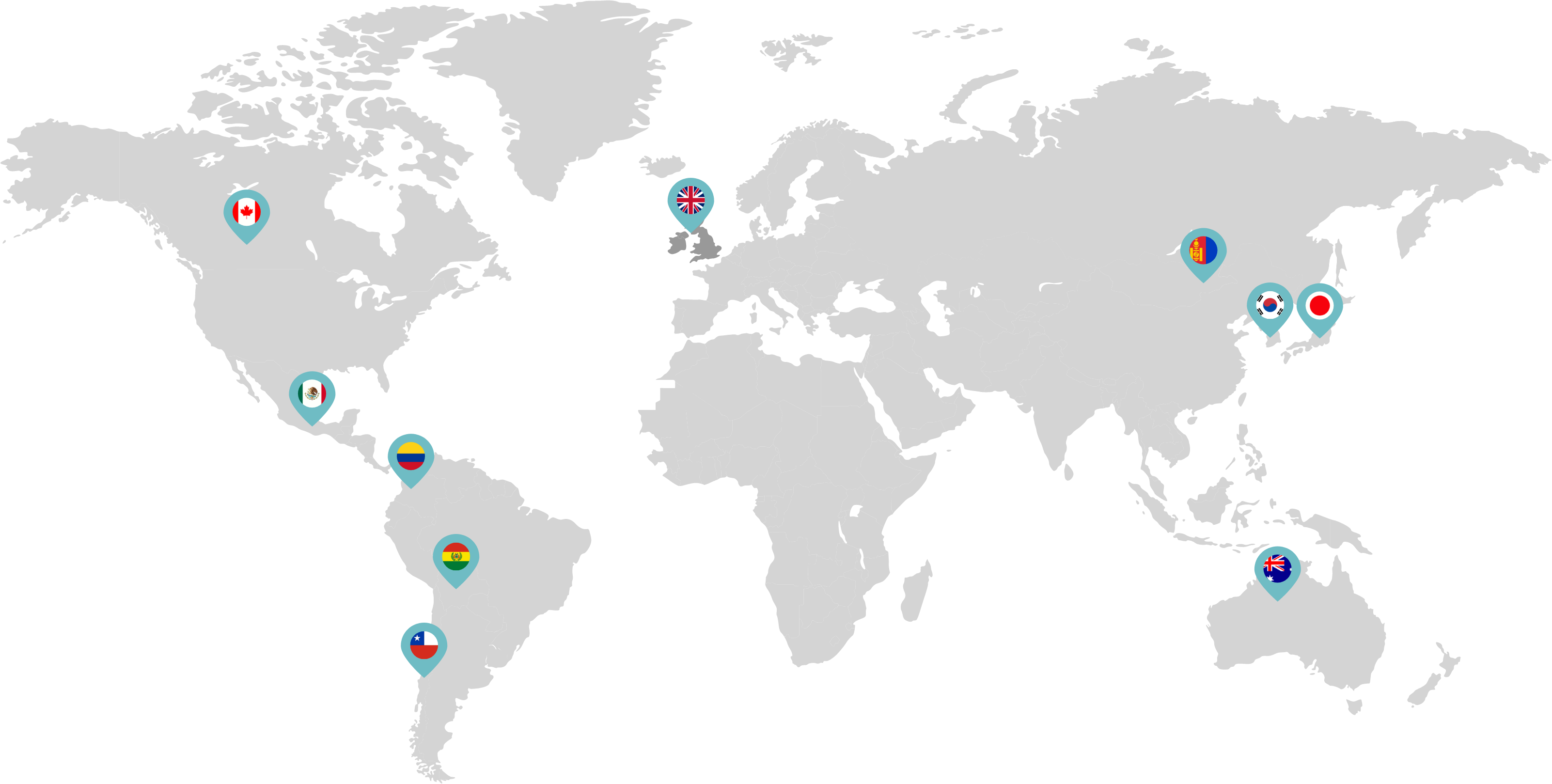
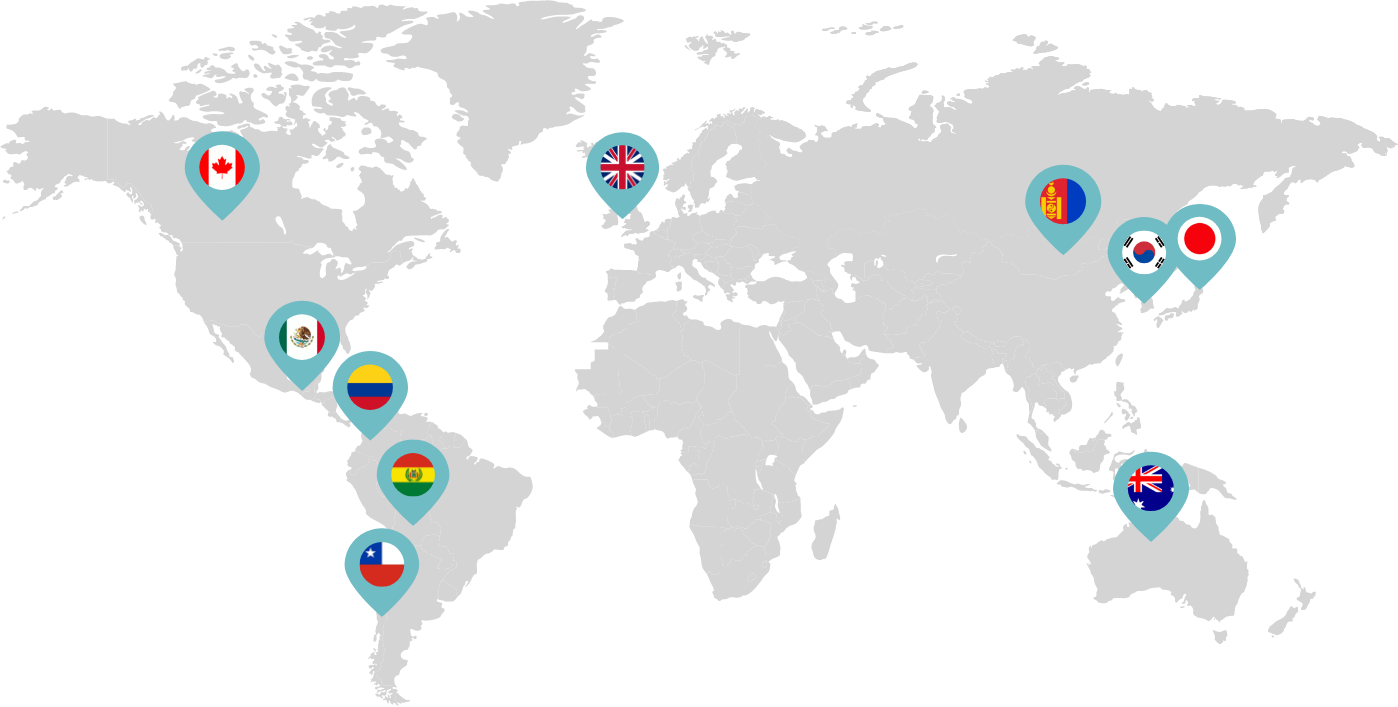
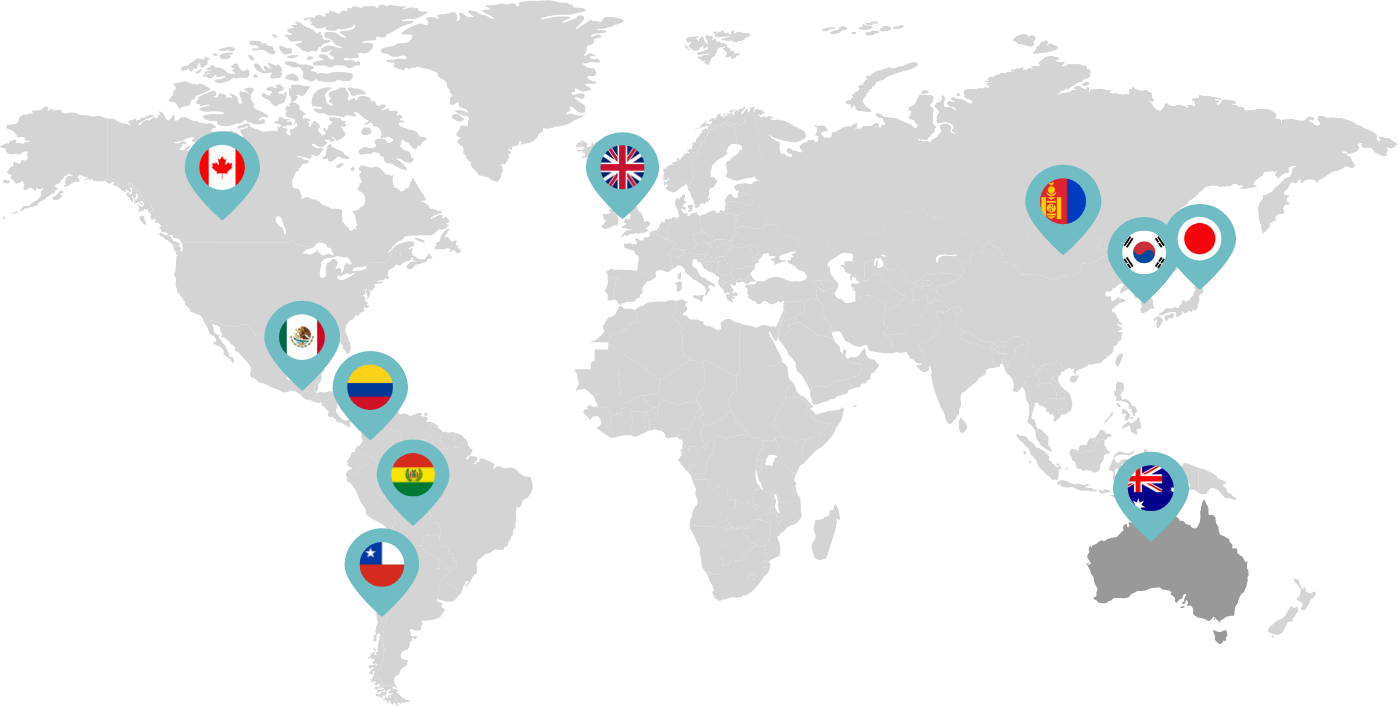
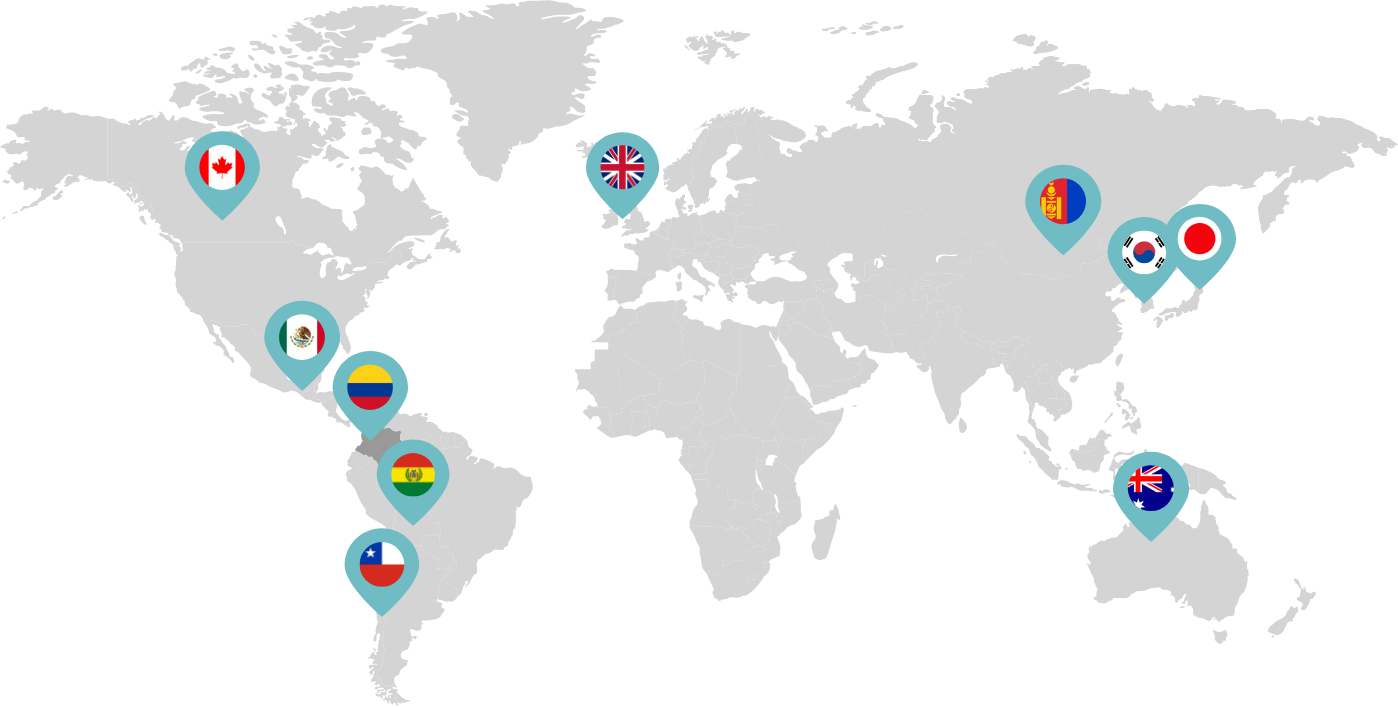
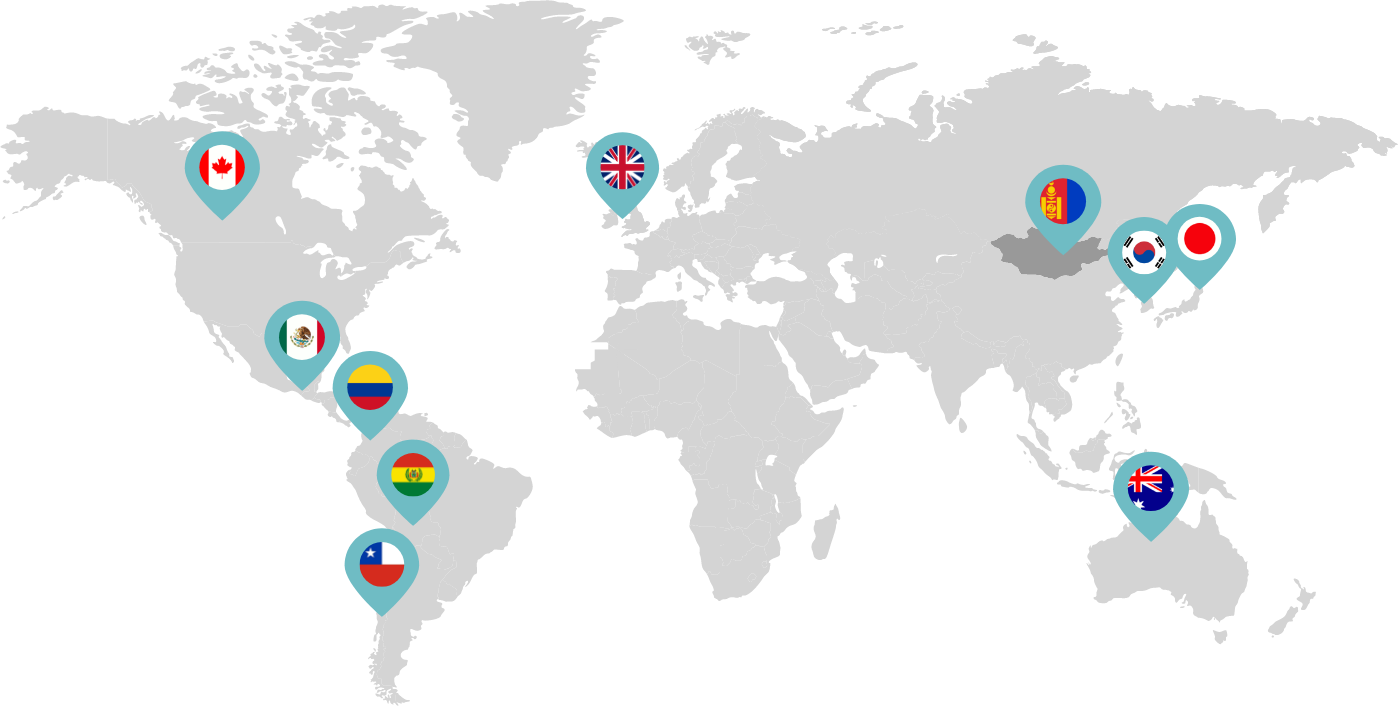
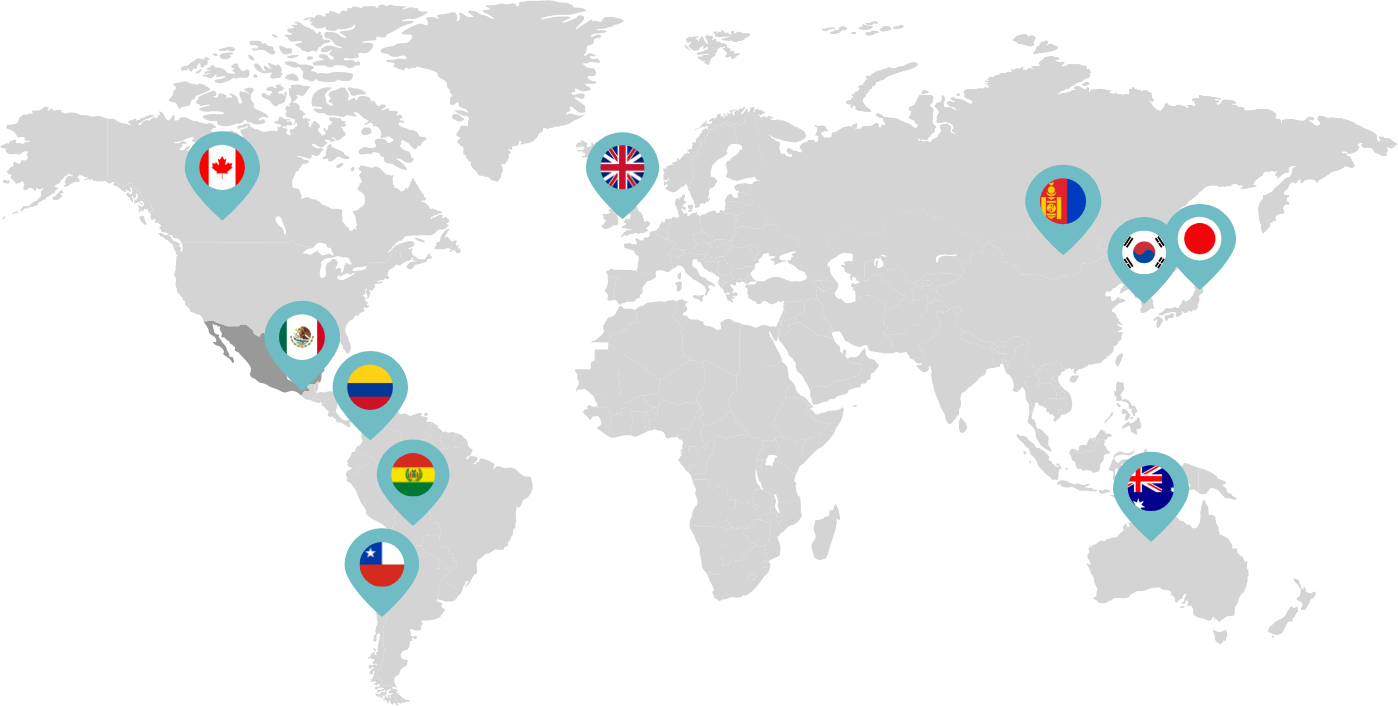
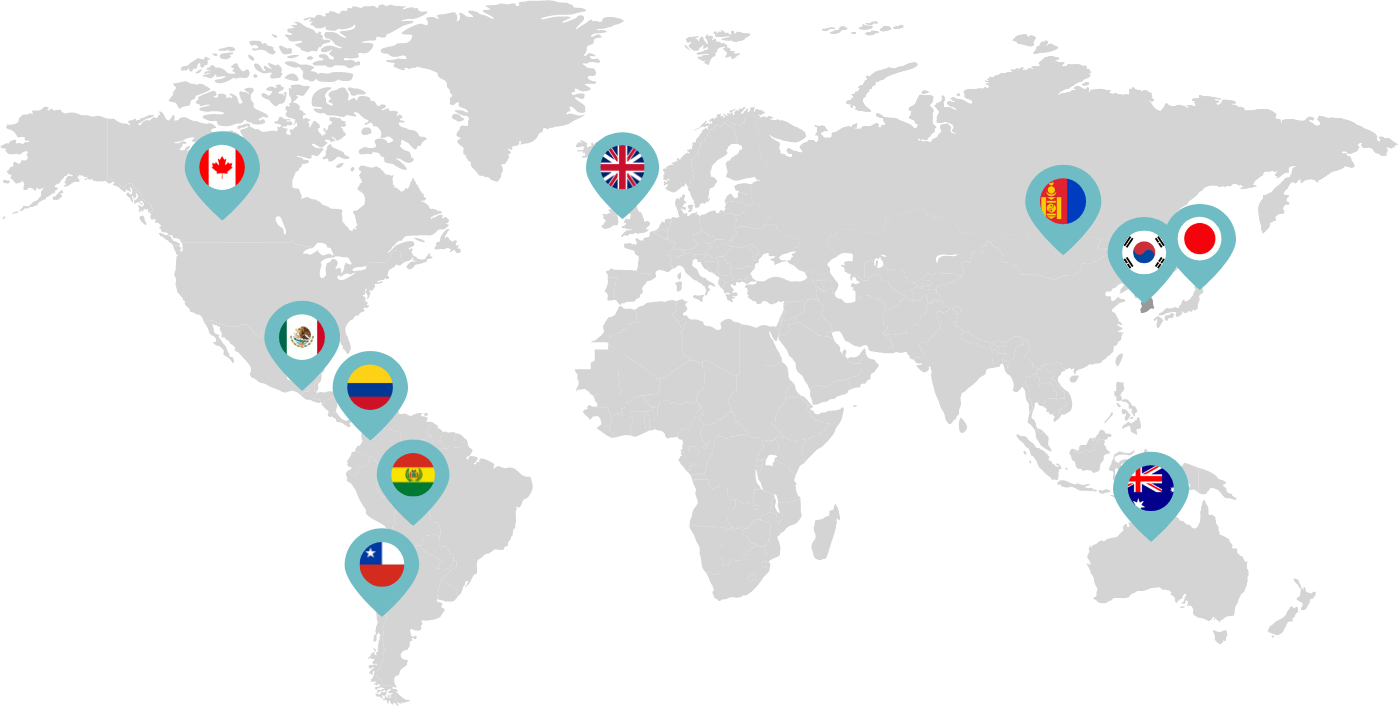
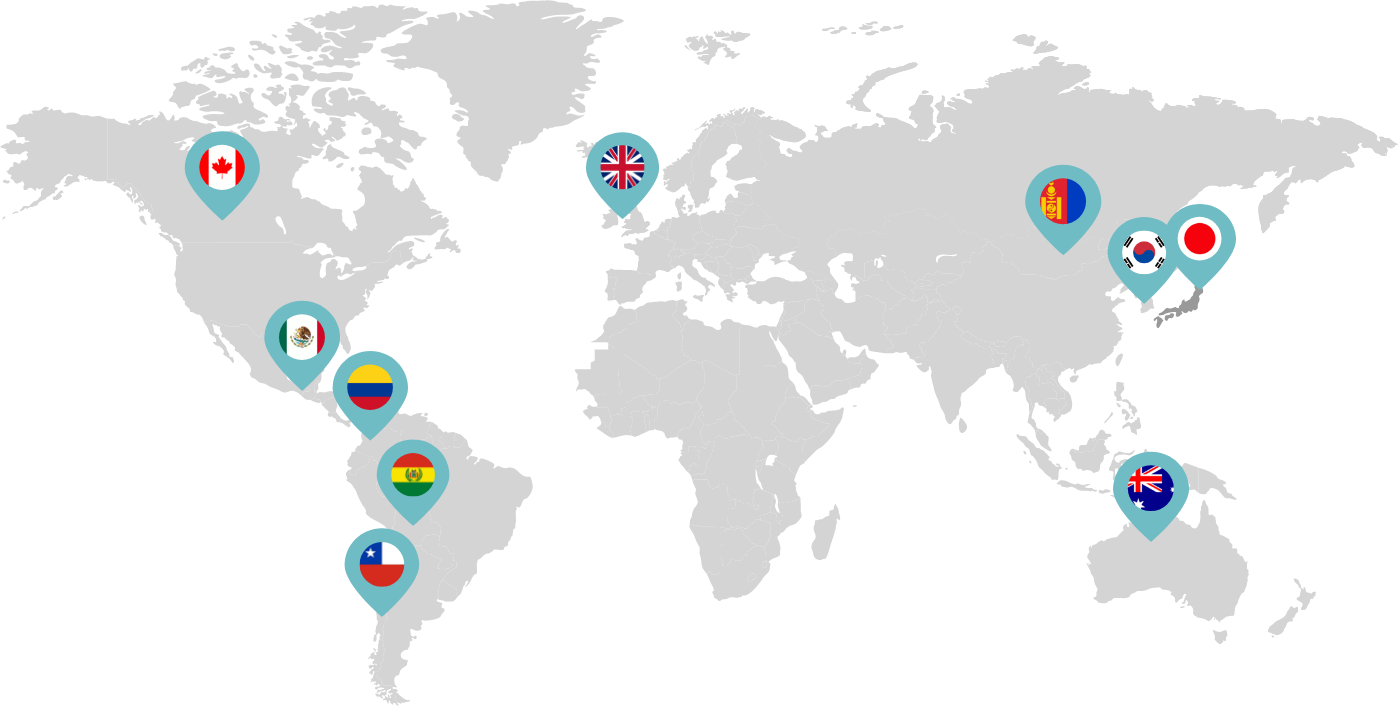
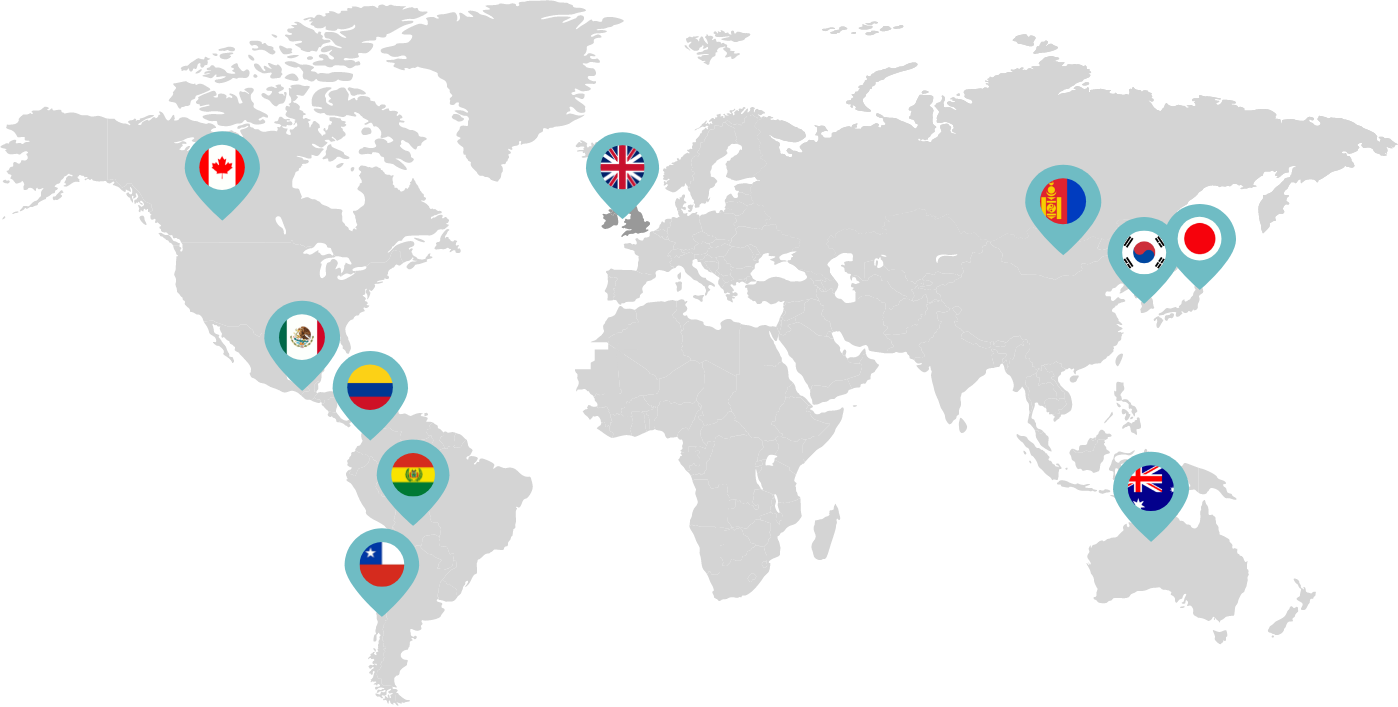
Primarily through partnerships with local distribution partners, we have commercialized mifepristone in over 25 countries worldwide. Initially a Euro-centric commercial focus, we now focus on expanding access to safe medication abortion and sexual and reproductive healthcare services and tools to the broader international market.
2010
Beginning in France, Linepharma International began providing high-quality non-surgical abortion products that meet the most rigorous regulatory health authorities standards to healthcare providers and their patients throughout Europe.
2014
After introducing single-packaged mifepristone in 2012, Australia became the first country to approve our co-packaged mifepristone and misoprostol product for the purposes of terminating a developing intrauterine pregnancy.
2015
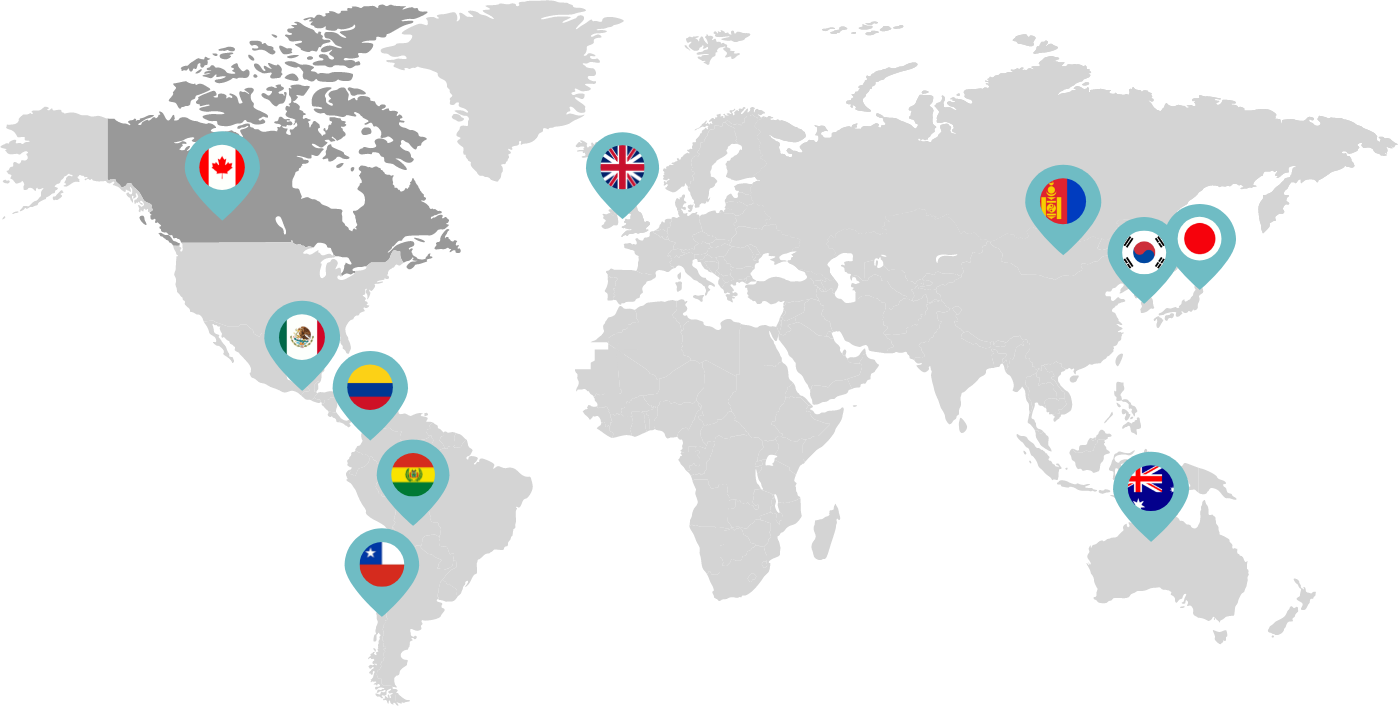
Canada became the second country to approve the co-packaged presentation of mifepristone and misoprostol.
2023
Japan became the third country to approve the co-packaged presentation of mifepristone and misoprostol.
Linepharma has successfully introduced mifepristone and misoprostol products for medication abortion in various countries throughout Asia and Latin America in partnership with local distributors while establishing full-scale pharmaceutical operations teams in key markets in Canada and Japan.
Linepharma International is headquartered in the United Kingdom and operates affiliate offices in Toronto, Canada, and Tokyo, Japan.
OUR VALUES
Access
We are dedicated to expanding sexual and reproductive health services and tools in new markets internationally while supporting their growth in existing markets.
Safety
We follow the most current scientific data and World Health Organization recommendations.
Support
We strive to improve patients’ and providers’ ability to access sexual and reproductive healthcare management tools.
Innovation
We are responsible for the world’s first co-packaged mifepristone and misoprostol developed specifically for medication abortion.
Quality
Products manufactured in Europe and approved by some of the most stringent health authorities.
OUR PARTNERS
Linepharma’s medication abortion products are approved by the world’s most stringent regulatory health authorities.
Our relationships with local distributors and non-governmental organizations in Australia, Canada, the United Kingdom, and parts of Asia and Latin America help bring medication abortion products to as many communities as possible.
Is medication abortion available in your country? If you’re a pharmaceutical distributor in the Americas or Asia-Pacific who may be interested in distributing Linepharma’s products, contact us today.